
We can visualize the ground state of an electron as a low orbit around an atomic nucleus and the higher energy state as a higher-level orbit. This interaction involves an electron being excited by the incident photon, causing it to jump from its relatively low energy level or ground state up to a higher energy state. Absorption occurs when incident photons interact with outermost electrons (such as valence electrons) within a chemical specimen. The method is based on the physics of electronic absorption.

Absorption SpectroscopyĪbsorption spectroscopy is a common analytical method that allows scientists to identify and study the properties of molecules in a chemical sample. This relationship between the energy and wavelength plays an important role in the processes that underly electronic absorption and emission spectroscopy.
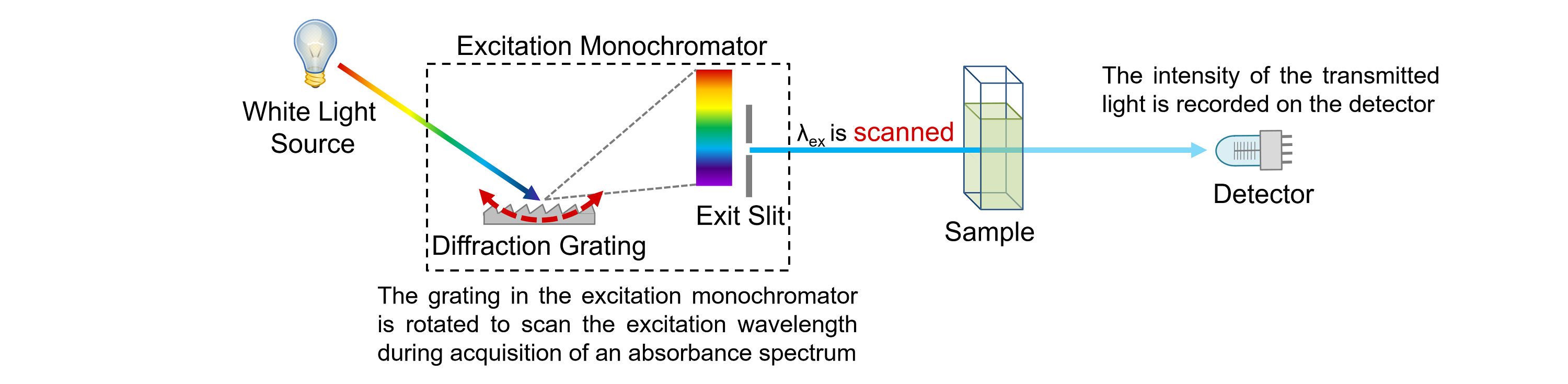
Therefore, short wavelength photons such as gamma rays and X-rays have more energy than long wavelength photons such as infrared light and radio waves. In this equation, we see that the wavelength is inversely proportional to the energy. The product of wavelength, \(𝛌\), and frequency, \(f\), for electromagnetic radiation is equal to the speed of light, \(c\). Electromagnetic radiation can be modeled as either propagating waves or as particle-like bundles of energy called photons.Įlectromagnetic radiation travels at the speed of light, and that speed also relates the wavelength and frequency of that radiation. While electromagnetic radiation is a continuous spectrum of energies, it is often grouped into categories including gamma rays, X-rays, ultraviolet light, visible light, infrared light, microwaves, and radio waves. Therefore, emission spectra can also be used to determine the chemical composition of materials.Įlectromagnetic Radiation: Wavelength and EnergyĮlectromagnetic radiation is a form of energy generated by coupled electric and magnetic fields oscillating. As with the absorption spectra, the wavelengths of emission lines are element-specific. The location of the lines indicates the wavelengths of light that are emitted from the sample. This image is an example of an emission spectrum. Therefore, absorption spectra can be used to determine the chemical composition of materials.Įmission spectra are made by measuring the wavelength and intensity of energy emitted from a specimen when heated or given excess energy in some form. The wavelengths of absorption lines are element-specific. The locations of the absorption lines indicate the specific wavelengths at which the substance absorbs incident photons. This image is an example of an absorption spectrum. Absorption spectra are produced by measuring the amount of electromagnetic radiation absorbed by a substance at a range of wavelengths from an incident light source.

The incident photon source is a form of electromagnetic radiation such as ultraviolet or visible light. More differences between absorption and emission spectrum are given below in a tabular column.Electronic absorption and emission spectra are graphical representations of the wavelengths of electromagnetic radiation that are absorbed or emitted by a particular substance. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the spectrum, whereas an absorption spectrum has dark-coloured lines in the spectrum. On the other hand, an absorption spectrum is constituted by the frequencies of light transmitted with dark bands when energy is absorbed by the electrons in the ground state to reach higher energy states. The emission spectrum is formed by the frequencies of these emitted light. These excited electrons have to radiate energy to return to ground states from the excited state, which is unstable. When energy is absorbed by electrons of an atom, electrons move from lower energy levels to higher energy levels. Learn their differences in-details given in the table provided below. These two topics are one of the most interesting concepts in Physics. The emission and absorption spectra difference is provided here.


 0 kommentar(er)
0 kommentar(er)
